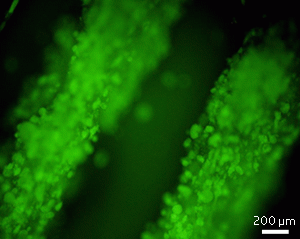
Pluripotent embryonic stem cells encapsulated in the fiber scaffold
developed at IBN.
A three-dimensional fiber scaffold promotes
large-scale stem cell proliferation and differentiation to levels suitable for
tissue transplants
Thanks to the ability of
pluripotent stem cells to self-renew and differentiate into a wide variety of
specialized cell types, they are expected to revolutionize the treatment of
illnesses such as type I diabetes and Parkinson’s disease. Before this becomes
a reality, however, scientists must develop culture systems to mass-produce
these cells. To overcome the limitations of previous single-layer-substrate
systems, a research team in Singapore has developed three-dimensional scaffolds
that stimulate stem cell proliferation and differentiation under defined
chemical conditions. Importantly, the system can be scaled up. The scaffolds
consist of microscopic fibers obtained by weaving together polymer strands
bearing opposite charges1.
Hongfang Lu and Andrew Wan from
the A*STAR Institute of Bioengineering and Nanotechnology led the research. Wan
notes that the fiber-based scaffold not only avoids the need to consume large
quantities of key growth factors, but it would also shield the cells from the
shear stresses generated in large-scale bioreactors.
To manufacture the scaffold, the
researchers opted for a positively charged biopolymer called chitin, which they
extracted from crab shell, and a negatively charged polymer called sodium
alginate. After depositing one droplet of each of these water-soluble polymers
onto a sterile substrate, they brought the droplet interfaces into contact
using forceps; this formed a chitin–alginate complex. Held together by
intermolecular electrostatic interactions, the complex extended into a
continuous fiber. The team reeled the fiber onto a holder to complete the
three-dimensional system.
By suspending the stem cells in
the alginate solution, Lu, Wan and co-workers incorporated the cells into the
scaffold during fiber formation, resulting in a network of uniformly
distributed cells (see image). Preliminary tests showed that when the
researchers destroyed the scaffold with enzymes, they could recover a high
number of the cells.
Lu explains that their system
provided a ‘micro-environment’ in which cells could grow in aggregates. When
sub-cultured over many generations, the encapsulated stem cells remained
pluripotent and did not undergo any genetic mutations. Moreover, the cells
displayed excellent viability when frozen in the fiber for storage; in
addition, they could either self-renew or differentiate, depending on the media
available to them. “The small dimensions of the fibers are useful because they
allow nutrients and growth factors to efficiently diffuse towards the cells within
the scaffold,” she adds.
The team is now planning to
exploit their approach to produce transplantable tissue for cell-based therapy.
“Our system allows us to generate large numbers of cells for tissue-engineering
applications,” says Wan.
The A*STAR-affiliated researchers
contributing to this research are from the Institute of Bioengineering and
Nanotechnology
References
- Lu, H. F., Narayanan, K., Lim, S.-X., Gao, S.,
Leong, M. F. & Wan, A. C. A. A 3D microfibrous scaffold for long-term
human pluripotent stem cell self-renewal under chemically defined
conditions. Biomaterials 33, 2419–2430
(2012). | article

No comments:
Post a Comment